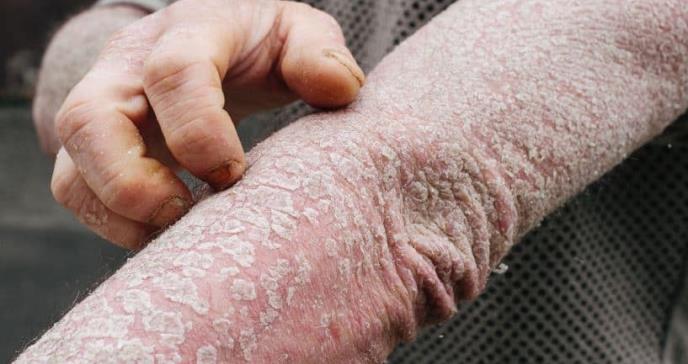
Antecedentes: Los medicamentos biológicos para la psoriasis en placas se han utilizado para tratar la psoriasis eritrodérmica (EP).
A total of 19 patients were administered secukinumab of whom 16 responded to treatment. Four of 8 cases that reported improvement between weeks 2-6 achieved PASI 75.47-49 One of these patients demonstrated continuous improvement and reached PASI 100 by weeks 8-12. Two additional patients showed PASI 100 within this time frame.47,50 Mateu-Puchades et al., also reported PASI scores during weeks 16-20 with 5/5 patients reaching PASI 90.48 Weng et al., described that 70% of patients responded to treatment showing evident clearing of psoriasis (PASI> 75) by week 16. One of these patients experienced relapse by week 24. Two patients demonstrated a sustained response after approximately 6 months.46
Infliximab
Forty-one patients were treated with infliximab. Seventeen had no PASI score reported but described marked improvement of erythroderma 2 weeks after infliximab infusion.20,22-25,31 One patient evaluated using a modified PASI score achieved significant response.18 In a study by Torii et al., eight patients were treated with infliximab and the median PASI improvement reported was 67.9% at week 10.27 Thus, we were unable to assess if any patient from the aforementioned study did not respond to therapy. Arroyo-Tridico and colleagues reported a patient who maintained PASI 100 for eleven years.17 Another study reported more than 90% improvement at week 6 in all 7 patients but did not specify which instrument was used to measure this response.19 Six other studies reported significant improvement in 10 patients by weeks 2-12 (PASI= 75).16,21,28-30,58 Poulalhon et al. reported PASI score-based outcomes by week 14: three patients achieved PASI= 75 and 2 patients did not respond.26
Adalimumab
Only three patients received adalimumab for EP. One patient showed remission at week 3 and the other two at week 12. None of them reported PASI scores.51-53
Etanercept
Fourteen patients were treated with etanercept. One patient did not report PASI scores but described significant improvement. Nevertheless, this patient relapsed during therapy.42 Twelve patients’ PASI scores were reported on weeks 8-12: seven achieved PASI= 75, three achieved PASI 50, and two did not respond.44,45,58 Romero-Mate et al. reported a patient with PASI 100 over 34 months.43
Ustekinumab
Fifty-four patients were administered ustekinumab. A total of sixteen cases reported PASI score improvement at weeks 2-6. Eight of these patients were not responsive during that time frame; all except one of the patients eventually responded to therapy.32,33,38-40 There were 44/46 cases with PASI= 50 16-24 weeks after starting medication.33-36,39,41 One patient did not respond to treatment; another patient initially responded but relapsed 28 weeks after intervention.39,41 Another case reported PASI= 90 at week 114 of treatment.37 Only 7.4% of patients failed to respond to treatment.34,39,41
Ixekizumab
Eight patients were treated with ixekizumab as part of an open-label study. By week 12, all patients achieved PASI 75, 5/8 achieved PASI 90, and 2/8 achieved PASI 100. By week 24, 100% of patients reached PASI 75, 7/8 reached PASI 90, and 1/8 reached PASI 100. PASI scores were sustained by week 52.54
Golimumab
One patient was treated with golimumab and responded to treatment by week 4 and continued to improve with PASI= 75 by week 12.55
Guselkumab
Guselkumab was reported in eleven patients and all responded. By week 8, all patients achieved PASI> 50. Fifty-two weeks after treatment, 10 patients demonstrated sustained responses (mean PASI= 75). One patient was lost to follow-up.56
Safety
Sixty (37.3%) adverse events (AE) were reported secondary to biologic therapy among studies that recorded their occurrence. Infectious events (35/60) were the most common. Further details can be found on Table III.
Discussion
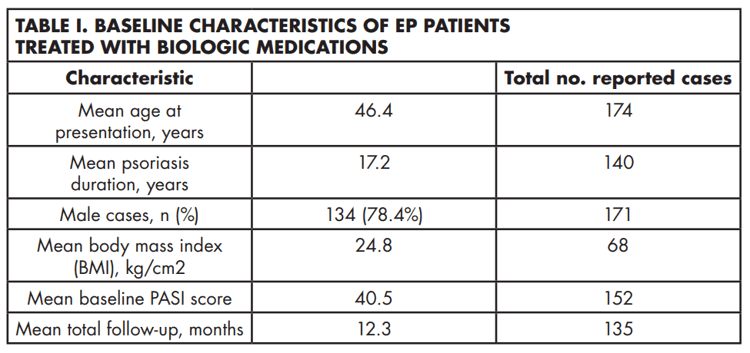
We aim to update the recommendations for treating EP with biologics published by Levin and colleagues in 2012.7 Since their publication, new biologics have been approved for the treatment of plaque psoriasis. There is a lack of randomized, double-blind, controlled trials, and head-to-head comparisons. Therefore, it is challenging to recommend any of the biologics as a first-line treatment for the management of EP. Recommendations for each biologic are depicted on Table II. Overall, ustekinumab was the most frequent biologic reported. It demonstrated a slower onset of action than infliximab. Nevertheless, patients treated with this medication reported longterm clinical improvement (follow-up period range 3-29 months) with few adverse events. These findings support the important role of IL-12 and IL-23 inflammatory pathways in the pathogenesis of EP.33 As reported by Stinco and colleagues, ustekinumab seems to be a promising candidate for the long-term control of EP, especially when taking into consideration the well-known possibility of developing tachyphylaxis while on infliximab.7,33
The second most common biologic used was infliximab, demonstrating excellent efficacy with few serious AE that cannot be fully attributed to the medication. The drug had a rapid onset of action, with responses achieved as early as 48 hours after initiation of treatment.22,24. Most patients demonstrated sustained improvement after 6-10 weeks.17,20,21,26-28 Infliximab was used in combination with methotrexate or acitretin in several cases but these patients failed to respond faster than those on monotherapy. 17,19-21,25,27 For these reasons, we agree with Rosenbach et al. and Levin et al., who suggested that infliximab should be considered a first-line therapeutic agent in the treatment of acute, severe, and/or unstable cases of EP.6,7
Secukinumab, an IL-17A inhibitor, was administered to 19 patients, of which 84.2% showed improvement by =8 weeks with no significant AE.46-48,50. However, 31.6% of patients suffered a relapse. Weng et al. attributed these recurrences to history of prior biologic failure in their patients.46 Although this may be a possibility, a proportion of the cases responsive to such agents had also been previously treated with alternate biologic medications. This implies that another mechanism could be responsible for relapses after treatment with secukinumab. Due to its high relapse rate, we recommend using secukinumab as second line therapy for EP based on individual patient characteristics.
Most patients treated with etanercept and adalimumab responded to therapy. Nevertheless, it is important to mention that 21 and 18 cases, respectively, who received other biologics reported prior failure to etanercept or adalimumab. Etanercept was discontinued in some cases mainly due to its lack of efficacy and AE.44,57 Consequently, we recommend considering etanercept and adalimumab as second-line treatments for EP.
Guselkumab (IL-23 inhibitor) and ixekizumab (IL-17 inhibitor) achieved significant improvement after 52 weeks of follow-up.54 Minor infections were a common AE of both medications. However, it is important to note that these studies had longer follow-up time periods when compared to most case reports and case series that reported on the response of other biologics. Due to their level of evidence both guselkumab and ixekizumab can be considered first line treatments for EP. Regardless of which medication is eventually selected for the treatment of EP, adjuvant measures including medium-potency topical steroids, moisturizers, wet dressings, oatmeal baths, and continued supportive care are important.6
Unfortunately, data is limited to case reports, case series, and uncontrolled studies. This could contribute to publication bias, as evidenced by the risk of bias assessment in which most studies had a moderate risk of bias. Furthermore, it is difficult to recommend a specific medication, and despite the suggested therapies it is important to treat every case on an individual basis.
In conclusion, biologic therapy in patients with EP seems to be well-tolerated and demonstrated positive response. Recommendations for biologic agent therapy in EP are based on limited evidence. We recommend infliximab or ustekinumab in acute, severe cases of EP as first-line biologic agents. IL-23 and IL-17 inhibitor agents seem to be a promising category of biologic treatment of EP and can also be considered as first-line therapy based on their level of evidence. Etanercept and adalimumab can be used in milder, biologically naïve cases. Therapy of patients with EP should be individualized and based on each patient’s disease characteristics, history of previous therapies, and comorbidities. Increased understanding of pathogenic mechanisms in EP and the continued development of newer biologic agents for the treatment of psoriasis will contribute to improve the quality of life in these seriously affected patients.
References
1.Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001 Jun;26(4):314-20.
2.Ladizinski B, Lee KC, Wilmer E, Alavi A, Mistry N, Sibbald RG. A review of the clinical variants and the management of psoriasis. Adv Skin Wound Care. 2013 Jun;26(6):271-84.
3.Lebwohl M. Psoriasis. Lancet. 2003 Apr 5; 361: 1197–204.
4.Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014 AprMay;13(4-5):490-5.
5.Singh RK, Lee KM, Ucmak D et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:93-104.
6.Rosenbach M, Hsu S, Korman NJ et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010 Apr;62(4):655-62.
7.Levin EC, Debbaneh M, Koo J, Liao W. Biologic therapy in erythrodermic and pustular psoriasis. J Drugs Dermatol. 2014 Mar;13(3):342-54.
8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3:e123-e130.
9.Haffar S, Bazerbachi F, Prokop L, Watt KD, Murad MH, Chari ST. Frequency and prognosis of acute pancreatitis associated with fulminant or non-fulminant acute hepatitis A: A systematic review. Pancreatology. 2017 Mar-Apr;17(2):166–175.
10.Wells G, Shea B, O’Connell D et al; The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa, Ontario: The Ottawa Health Research Institute, 2011.
11. Bazerbachi F, Haffar S, Szarka LA et al. Secretory diarrhea and hypokalemia associated with colonic pseudo-obstruction: A case study and systematic analysis of the literature. Neurogastroenterol Motil. 2017 Nov 29(11).
12.Bazerbachi F, Sawas T, Vargas EJ et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis. Gastrointest Endosc. 2018 Jan;87(1):30- 42.
13.Bazerbachi F, Leise MD, Watt KD, Murad MH, Prokop LJ, Haffar S. Systematic review of mixed cryoglobulinemia associated with hepatitis E virus infection: asab, etanercept, ustekinumab, and adalimumab. Articles written in Spanish and English were included. There were no limitations on article type. After the selection process, the references of all included articles were assessed for missing publications. The review protocol is registered with PROSPERO (CRD42019133413) (https://www. crd.york.ac.uk/prospero/).
Study eligibility, selection criteria and screening
Independent assessment of the titles and abstracts was carried out independently by two of the authors (O.Y.C. and K.J.C.). Disagreements were resolved through discussion with a third author (R.F.M.). All studies reporting one or more cases of EP treated with biologics were included. EP was defined as >75% of body surface area involvement with inflammatory erythema and scaling at baseline. The following criteria were used to exclude articles: pediatric patients (<18 years); <75% of body surface area involvement; erythroderma caused by a condition other than psoriasis; patient was treated with biologics for a different condition that was not EP; article failed to mention response to treatment. If the full text was not available online, it was ordered at the University of Puerto Rico School of Medicine library.
Data extraction and statistical analysis The following variables were gathered as available: age, sex, body mass index, duration of disease, comorbidities, prior therapies, adjuvant treatments, Psoriasis Area and Severity Index (PASI) score, total follow-up time, PASI score improvement, and adverse events. Adequate response to treatment was defined as PASI= 50. Statistical analysis was done using IBM SPSS version 25.
Risk of bias, level of evidence, and grade of recommendation assessment
Due to the nature of the condition, we did not expect to find comparative studies. To assess the risk of bias of studies, we used a tool developed by Haffar et al. derived from the New-Castle Ottawa scale.9,10 This tool has been used previously.9,11-14 It consists of 5 criteria in the form of questions with a binary response (yes/no) to indicate whether or not the item is suggestive of bias. The tool is composed of the following questions:
1.Did the patient(s) represent the whole case(s) of the medical center?
2.Was the diagnosis correctly made?
3.Were other important diagnoses excluded?
4.Were all important data cited in the report?
5.Was the outcome correctly ascertained?
Quality of the report was considered good (low risk of bias) when all 5 criteria were fulfilled, moderate (moderate risk of bias) when 4 were fulfilled, and poor (high risk of bias) when = 3 were fulfilled. Two of the authors (O.Y.C. and G.P.) assessed the risk of bias of the studies with discussion with a third author (R.F.M.) in case of disagreement. Recommendations for each biologic were given based on criteria by Robinson and colleagues.15
RESULTS
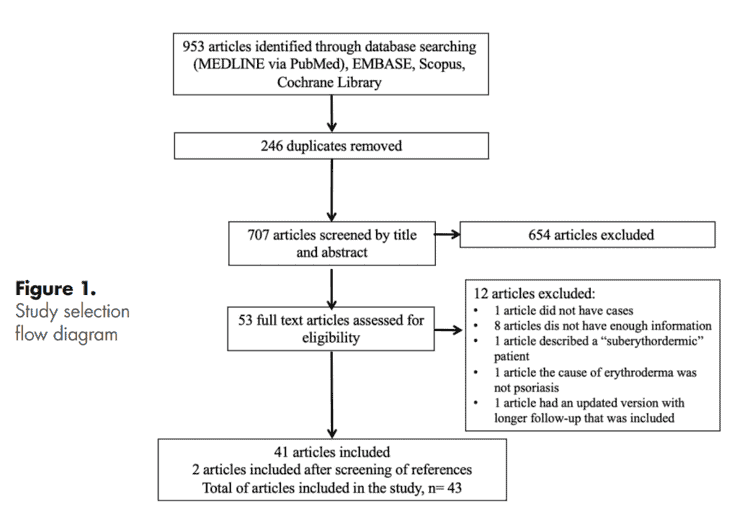
Systematic search results Forty-three articles were included, yielding a total of 179 patients of EP treated with biologic medications (Figure 1). The majority of the articles were case reports (65.1%), followed by case series (14.0%) and open label studies (11.6%). There were 3 retrospective and 1 open prospective study. Sixteen articles reported treatment with infliximab16-31, 10 ustekinumab32-41, 4 etanercept42-45, 5 secukinumab46-50, and 3 adalimumab51-53. Ixekizumab54, golimumab55, and guselkumab56 were each found in one study. One article reported cases treated with either infliximab, adalimumab, etanercept or ustekinumab, and another article reported cases treated with infliximab or etanercept.57,58
Description of cases The mean age at presentation was 46.4 years with a male-to-female ratio of 3.6:1. Table 1 shows baseline characteristics of patients. After assessing all the selected articles, the most common medications reported were ustekinumab and infliximab with 54 and 41 cases, respectively.16-41,58 They were followed by secukinumab (n=19), etanercept (n=14), guselkumab (n=11), ixekizumab (n= 8), adalimumab (n=3), and golimumab (n=1).42-56,58 One article described 42 flares in 28 patients treated with multiple biologic agents (infliximab, adalimumab, etanercept, and ustekinumab) and was not included in the aforementioned analysis as data for individual patients could not be extracted.57 Supplemental Table I shows a summary of the studies where EP was treated with biologics and risk of bias assessment.
Efficacy
The majority of patients showed adequate response in their condition at some point during treatment intervention. Out of 122 patients that reported PASI scores following treatment with biologics, 9.0% of patients failed to achieve PASI 50.26,34,39,46 Furthermore, in a multicenter retrospective study with multiple biologics, Viguier and colleagues57 reported PASI 75 at 10-14 weeks in 40% of those treated with infliximab, and equal improvement in 67%, 67%, and 0% of patients treated with etanercept, adalimumab, and ustekinumab, respectively. A smaller proportion of patients maintained PASI 75 at weeks 22-24 (infliximab 20%; etanercept 50%; adalimumab 60%). Below there is a summary of the efficacy of each biologic medication in the treatment of EP based on reduction of PASI score. Viguier’s study was excluded from the following analysis as the article reported flares instead of patients and thus, we were unable to extract the individual data from the study. Recommendations for each biologic are depicted on Table 2.
Secukinumabsociation or causation?Gastroenterol Rep. 2017;5:178–84.
14.Bazerbachi F, Haffar S, Hussain MT et al. Systematic review of acute pancreatitis associated with interferon- or pegylated interferon- : possible or definitive causation? Pancreatology. 2018 Oct;18(7):691- 699.
15.Robinson JK, Dellavalle RP, Bigby M, Callen JP. Systematic reviews: grading recommendations and evidence quality. Arch Dermatol. 2008 Jan;144(1):97-9.
16.Valdés AM del P, Schroeder HF, Roizen GV, Honeyman MJ, Sánchez ML. [Efficacy of infliximab in patients with moderate and severe psoriasis treated with infliximab (Remicade)]. Rev Med Chil. 2006 Mar;134(3):326-31.
17.Arroyo-Trídico L, Antonio JR, Mathias CE, Pozetti EMO. Effectiveness and safety of infliximab for 11 years in a patient with erythrodermic psoriasis and psoriatic arthritis. An Bras Dermatol. 2017 SepOct;92(5):743-745.
18.Rongioletti F, Borenstein M, Kirsner R, Kerdel F. Erythrodermic, recalcitrant psoriasis: clinical resolution with infliximab. J Dermatolog Treat. 2003 Dec;14(4):222-5.
19. Takahashi MD, Castro LG, Romiti R. Infliximab, as sole or combined therapy, induces rapid clearing of erythrodermic psoriasis. Br J Dermatol. 2007 Oct;157(4):828- 31.
20.Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;85(3):247-8.
21.Yip L, Harrison S, Foley P. From biologic to biologic to biologic: lessons to learn for erythrodermic and recalcitrant chronic plaque psoriasis. Australas J Dermatol. 2008 Aug;49(3):152-5.
22.O’Quinn RP, Miller JL. The effectiveness of tumor necrosis factor alpha antibody (infliximab) in treating recalcitrant psoriasis: a report of 2 cases. Arch Dermatol. 2002 May;138(5):644-8.
23.Fiehn C, Andrassy K. Case number 29: hitting three with one strike: rapid improvement of psoriatic arthritis, psoriatic erythroderma, and secondary renal amyloidosis by treatment with infliximab (Remicade). Ann Rheum Dis. 2004 Mar;63(3):232.
24.Lewis TG, Tuchinda C, Lim HW, Wong HK. Life-threatening pustular and erythrodermic psoriasis responding to infliximab. J Drugs Dermatol. 2006 Jun;5(6):546-8. 25.Heikkilä H, Ranki A, Cajanus S, Karvonen SL. Infliximab combined with methotrexate as long-term treatment for erythroRevista Puertorriqueña de Medicina y Salud Pública 31 dermic psoriasis. Arch Dermatol. 2005 Dec;141(12):1607-10. 26.Poulalhon N, Begon E, Lebbé C et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007 Feb;156(2):329-36. 27.Torii H, Nakagawa H. Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011 Apr;38(4):321-34.
28. Kurokawa R, Hagiwara A, Niijima Y, Kojima K. Computed tomography imaging findings in erythrodermic psoriasis treated with infliximab: A case report. Radiol Case Rep. 2018 Mar 2;13(2):460-463.
29.Belinchon I, Lucas A, Ballester I, Betlloch I, Pérez-Crespo M: Successful treatment of life-threatening erythrodermic psoriasis with infliximab. J Am Acad Dermatol. 2009, 60(3):AB171.
30.Yip L, Harrison S, Foley P: Infliximab rescue of efalizumab withdrawal flare and psoriasis-precipitated depression. Australas J Dermatol. 2008, 49(4):250-251.
31.Suárez Pedreira I, Santos Juanes J, Caminal Montero L, Trapiella L: Infliximab: An alternative in refractory erythrodermic psoriasis. Piel. 2006, 21(6):317-318.
32.Saraceno R, Talamonti M, Galluzzo M, Chiricozzi A, Costanzo A, Chimenti S. Ustekinumab treatment of erythrodermic psoriasis occurring after physical stress: a report of two cases. Case Rep Dermatol. 2013 Sep 26;5(3):254-9.
33.Stinco G, Piccirillo A, Errichetti E, Bergamo S, Patrone P. Treatment of Recalcitrant Erythrodermic Psoriasis With Ustekinumab. Eur J Dermatol. 2014 MayJun;24(3):387-90.
34.Pescitelli L, Dini V, Gisondi P et al. Erythrodermic psoriasis treated with ustekinumab: an Italian multicenter retrospective analysis. J Dermatol Sci. 2015 May;78(2):149-51.
35.Kim YS, Kim HJ, Lee S, Park YL. Erythrodermic Psoriasis Improved by Ustekinumab: A Report of Two Cases. Ann Dermatol. 2016 Feb;28(1):121-2.
36.Koutsoukou XA, Papadavid E, Theodoropoulos K, Rigopoulos D. Ustekinumab in severe complicated erythrodermic psoriasis: rapid clearing, safety, and sustained remission. Dermatol Ther. 2014 SepOct;27(5):257-9.
37. Castiñeiras I, Fernández-Diaz L, Juárez Y, Lueiro M. Sustained efficacy of ustekinumab in refractory erythrodermic psoriasis after failure of antitumor necrosis factor therapies. J Dermatol. 2012 Aug;39(8):730-1.
38.Santos-Juanes J, Coto-Segura P, Mas-Vidal A, Galache Osuna C. Ustekinumab induces rapid clearing of erythrodermic psoriasis after failure of antitumour necrosis factor therapies. Br J Dermatol. 2010 May;162(5):1144-6.
39.Wang TS, Tsai TF. Clinical experience of ustekinumab in the treatment of erythrodermic psoriasis: a case series. J Dermatol. 2011 Nov;38(11):1096-9.
40.Errichetti E, Piccirillo A: Latent tuberculosis reactivation in a patient with erythrodermic psoriasis under treatment with ustekinumab and a low dose steroid, Despite isoniazid chemoprophylaxis. Eur J Dermatol. 2014, 24(4):508-509.
41.Concha-Garzón MJ, Godoy-Trapero A, Daudén E, et al. Short- and long-term treatment of erythrodermic psoriasis with ustekinumab: A national and multicenter case series. J Am Acad Dermatol. 2014, 70(5):AB189.
42.Talat H, Wahid Z, Feroz F, Sajid M. Erythrodermic Psoriasis and Hepatitis C Infection Treated with Pegylated Interferon and Anti-TNF (Etanercept) Therapy. J Coll Physicians Surg Pak. 2017 Sep;27(9):S77-S79.
43.Romero-Maté A, García-Donoso C, Martinez-Morán C, Hernández-Núñez A, Borbujo J.Long-term management of erythrodermic psoriasis with anti-TNF agents. Dermatol Online J. 2010 Jun 15;16(6):15.
44. Esposito M, Mazzotta A, de Felice C, Papoutsaki M, Chimenti S. Treatment of erythrodermic psoriasis with etanercept. Br J Dermatol. 2006 Jul;155(1):156-9.
45. Piqué-Duran E, Pérez-Cejudo JA. [Psoriatic erythroderma treated with etanercept]. Actas Dermosifiliogr. 2007 Sep;98(7):508-10.
46.Weng HJ, Wang TS, Tsai TF. Clinical experience of secukinumab in the treatment of erythrodermic psoriasis: a case series. Br J Dermatol. 2018 Jun;178(6):1439-1440.
47.Mugheddu C, Atzori L, Lappi A, Pau M, Murgia S, Rongioletti F. Successful Secukinumab treatment of generalized pustular psoriasis and erythrodermic psoriasis. J Eur Acad Dermatol Venereol. 2017 Sep;31(9):e420-e421.
48.Mateu-Puchades A, Santos-Alarcón S, Martorell-Calatayud A, Pujol-Marco C, Sánchez-Carazo JL. Erythrodermic psoriasis and secukinumab: Our clinical experience. Dermatol Ther. 2018 Jul;31(4):e12607.
49.Galluzzo M, D’Adamio S, Campione E, Mazzilli S, Bianchi L, Talamonti M. A clinical case of severe disease burden: an erythrodermic psoriatic patient treated with secukinumab. J Dermatolog Treat. 2018 Sep 26:1-11.
50.Rongioletti F, Mugheddu C, Murgia S: Repigmentation and new growth of hairs after anti–interleukin-17 therapy with secukinumab for psoriasis. JAAD Case Rep. 2018 Jun; 4(5): 486–488.
51.Mumoli N, Vitale J, Gambaccini L, Sabatini S, Brondi B, Cei M. Erythrodermic psoriasis. QJM. 2014 Apr;107(4):315.
52.Richetta AG, Maiani E, Carlomagno V et al. Treatment of erythrodermic psoriasis in HCV+ patient with adalimumab. Dermatol Ther. 2009 Nov;22 Suppl 1:S16-8.
53.Vidal D, Peramiquel L, Olivella R, Villar M, Petiti G, González A: Erythrodermic psoriasis successfully treated with adalimumab: A case study. J Eur Acad Dermatol Venereol. 2013, 27(S4):68.
54.Saeki H, Nakagawa H, Nakajo K et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: Results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017 Apr;44(4):355-362.
55.Lee WK, Kim GW, Hyun-Ho Cho et al. Erythrodermic Psoriasis Treated with Golimumab: A Case Report. Ann Dermatol 2015;27(4): 446-449.
56.Sano S, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: Efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018 May;45(5):529-539.
57.Viguier M, Pagès C, Aubin F et al. Efficacy and safety of biologics in erythrodermic psoriasis: a multicentre, retrospective study. Br J Dermatol. 2012 Aug;167(2):417- 23.
58.Sahel H, Otsmane F, Bouadjar B: Treatment of erythrodermic psoriasis with biological therapies in two cases. J Eur Acad Dermatol Venereol. 2016, 30(S6):102.
after initiation of treatment.22,24. Most patients demonstrated sustained improvement after 6-10 weeks.17,20,21,26-28 Infliximab was used in combination with methotrexate or acitretin in several cases but these patients failed to respond faster than those on monotherapy. 17,19-21,25,27 For these reasons, we agree with Rosenbach et al. and Levin et al., who suggested that infliximab should be considered a first-line therapeutic agent in the treatment of acute, severe, and/or unstable cases of EP.6,7
Secukinumab, an IL-17A inhibitor, was administered to 19 patients, of which 84.2% showed improvement by =8 weeks with no significant AE.46-48,50. However, 31.6% of patients suffered a relapse. Weng et al. attributed these recurrences to history of prior biologic failure in their patients.46 Although this may be a possibility, a proportion of the cases responsive to such agents had also been previously treated with alternate biologic medications. This implies that another mechanism could be responsible for relapses after treatment with secukinumab. Due to its high relapse rate, we recommend using secukinumab as second line therapy for EP based on individual patient characteristics.
Most patients treated with etanercept and adalimumab responded to therapy. Nevertheless, it is important to mention that 21 and 18 cases, respectively, who received other biologics reported prior failure to etanercept or adalimumab. Etanercept was discontinued in some cases mainly due to its lack of efficacy and AE.44,57 Consequently, we recommend considering etanercept and adalimumab as second-line treatments for EP.
Guselkumab (IL-23 inhibitor) and ixekizumab (IL-17 inhibitor) achieved significant improvement after 52 weeks of follow-up.54 Minor infections were a common AE of both medications. However, it is important to note that these studies had longer follow-up time periods when compared to most case reports and case series that reported on the response of other biologics. Due to their level of evidence both guselkumab and ixekizumab can be considered first line treatments for EP. Regardless of which medication is eventually selected for the treatment of EP, adjuvant measures including medium-potency topical steroids, moisturizers, wet dressings, oatmeal baths, and continued supportive care are important.6
Unfortunately, data is limited to case reports, case series, and uncontrolled studies. This could contribute to publication bias, as evidenced by the risk of bias assessment in which most studies had a moderate risk of bias. Furthermore, it is difficult to recommend a specific medication, and despite the suggested therapies it is important to treat every case on an individual basis.
In conclusion, biologic therapy in patients with EP seems to be well-tolerated and demonstrated positive response. Recommendations for biologic agent therapy in EP are based on limited evidence. We recommend infliximab or ustekinumab in acute, severe cases of EP as first-line biologic agents. IL-23 and IL-17 inhibitor agents seem to be a promising category of biologic treatment of EP and can also be considered as first-line therapy based on their level of evidence. Etanercept and adalimumab can be used in milder, biologically naïve cases. Therapy of patients with EP should be individualized and based on each patient’s disease characteristics, history of previous therapies, and comorbidities. Increased understanding of pathogenic mechanisms in EP and the continued development of newer biologic agents for the treatment of psoriasis will contribute to improve the quality of life in these seriously affected patients.
References
1.Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001 Jun;26(4):314-20.
2.Ladizinski B, Lee KC, Wilmer E, Alavi A, Mistry N, Sibbald RG. A review of the clinical variants and the management of psoriasis. Adv Skin Wound Care. 2013 Jun;26(6):271-84.
3.Lebwohl M. Psoriasis. Lancet. 2003 Apr 5; 361: 1197–204.
4.Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014 AprMay;13(4-5):490-5.
5.Singh RK, Lee KM, Ucmak D et al. Erythrodermic psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:93-104.
6.Rosenbach M, Hsu S, Korman NJ et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010 Apr;62(4):655-62.
7.Levin EC, Debbaneh M, Koo J, Liao W. Biologic therapy in erythrodermic and pustular psoriasis. J Drugs Dermatol. 2014 Mar;13(3):342-54.
8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3:e123-e130.
9.Haffar S, Bazerbachi F, Prokop L, Watt KD, Murad MH, Chari ST. Frequency and prognosis of acute pancreatitis associated with fulminant or non-fulminant acute hepatitis A: A systematic review. Pancreatology. 2017 Mar-Apr;17(2):166–175.
10.Wells G, Shea B, O’Connell D et al; The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa, Ontario: The Ottawa Health Research Institute, 2011.
11. Bazerbachi F, Haffar S, Szarka LA et al. Secretory diarrhea and hypokalemia associated with colonic pseudo-obstruction: A case study and systematic analysis of the literature. Neurogastroenterol Motil. 2017 Nov 29(11).
12.Bazerbachi F, Sawas T, Vargas EJ et al. Metal stents versus plastic stents for the management of pancreatic walled-off necrosis: a systematic review and meta-analysis. Gastrointest Endosc. 2018 Jan;87(1):30- 42.
13.Bazerbachi F, Leise MD, Watt KD, Murad MH, Prokop LJ, Haffar S. Systematic review of mixed cryoglobulinemia associated with hepatitis E virus infection: association or causation?Gastroenterol Rep. 2017;5:178–84.
14.Bazerbachi F, Haffar S, Hussain MT et al. Systematic review of acute pancreatitis associated with interferon- or pegylated interferon- : possible or definitive causation? Pancreatology. 2018 Oct;18(7):691- 699.
15.Robinson JK, Dellavalle RP, Bigby M, Callen JP. Systematic reviews: grading recommendations and evidence quality. Arch Dermatol. 2008 Jan;144(1):97-9.
16.Valdés AM del P, Schroeder HF, Roizen GV, Honeyman MJ, Sánchez ML. [Efficacy of infliximab in patients with moderate and severe psoriasis treated with infliximab (Remicade)]. Rev Med Chil. 2006 Mar;134(3):326-31.
17.Arroyo-Trídico L, Antonio JR, Mathias CE, Pozetti EMO. Effectiveness and safety of infliximab for 11 years in a patient with erythrodermic psoriasis and psoriatic arthritis. An Bras Dermatol. 2017 SepOct;92(5):743-745.
18.Rongioletti F, Borenstein M, Kirsner R, Kerdel F. Erythrodermic, recalcitrant psoriasis: clinical resolution with infliximab. J Dermatolog Treat. 2003 Dec;14(4):222-5.
19. Takahashi MD, Castro LG, Romiti R. Infliximab, as sole or combined therapy, induces rapid clearing of erythrodermic psoriasis. Br J Dermatol. 2007 Oct;157(4):828- 31.
20.Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;85(3):247-8.
21.Yip L, Harrison S, Foley P. From biologic to biologic to biologic: lessons to learn for erythrodermic and recalcitrant chronic plaque psoriasis. Australas J Dermatol. 2008 Aug;49(3):152-5.
22.O’Quinn RP, Miller JL. The effectiveness of tumor necrosis factor alpha antibody (infliximab) in treating recalcitrant psoriasis: a report of 2 cases. Arch Dermatol. 2002 May;138(5):644-8.
23.Fiehn C, Andrassy K. Case number 29: hitting three with one strike: rapid improvement of psoriatic arthritis, psoriatic erythroderma, and secondary renal amyloidosis by treatment with infliximab (Remicade). Ann Rheum Dis. 2004 Mar;63(3):232.
24.Lewis TG, Tuchinda C, Lim HW, Wong HK. Life-threatening pustular and erythrodermic psoriasis responding to infliximab. J Drugs Dermatol. 2006 Jun;5(6):546-8. 25.Heikkilä H, Ranki A, Cajanus S, Karvonen SL. Infliximab combined with methotrexate as long-term treatment for erythroRevista Puertorriqueña de Medicina y Salud Pública 31 dermic psoriasis. Arch Dermatol. 2005 Dec;141(12):1607-10. 26.Poulalhon N, Begon E, Lebbé C et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007 Feb;156(2):329-36. 27.Torii H, Nakagawa H. Long-term study of infliximab in Japanese patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis and psoriatic erythroderma. J Dermatol. 2011 Apr;38(4):321-34.
28. Kurokawa R, Hagiwara A, Niijima Y, Kojima K. Computed tomography imaging findings in erythrodermic psoriasis treated with infliximab: A case report. Radiol Case Rep. 2018 Mar 2;13(2):460-463.
29.Belinchon I, Lucas A, Ballester I, Betlloch I, Pérez-Crespo M: Successful treatment of life-threatening erythrodermic psoriasis with infliximab. J Am Acad Dermatol. 2009, 60(3):AB171.
30.Yip L, Harrison S, Foley P: Infliximab rescue of efalizumab withdrawal flare and psoriasis-precipitated depression. Australas J Dermatol. 2008, 49(4):250-251.
31.Suárez Pedreira I, Santos Juanes J, Caminal Montero L, Trapiella L: Infliximab: An alternative in refractory erythrodermic psoriasis. Piel. 2006, 21(6):317-318.
32.Saraceno R, Talamonti M, Galluzzo M, Chiricozzi A, Costanzo A, Chimenti S. Ustekinumab treatment of erythrodermic psoriasis occurring after physical stress: a report of two cases. Case Rep Dermatol. 2013 Sep 26;5(3):254-9.
33.Stinco G, Piccirillo A, Errichetti E, Bergamo S, Patrone P. Treatment of Recalcitrant Erythrodermic Psoriasis With Ustekinumab. Eur J Dermatol. 2014 MayJun;24(3):387-90.
34.Pescitelli L, Dini V, Gisondi P et al. Erythrodermic psoriasis treated with ustekinumab: an Italian multicenter retrospective analysis. J Dermatol Sci. 2015 May;78(2):149-51.
35.Kim YS, Kim HJ, Lee S, Park YL. Erythrodermic Psoriasis Improved by Ustekinumab: A Report of Two Cases. Ann Dermatol. 2016 Feb;28(1):121-2.
36.Koutsoukou XA, Papadavid E, Theodoropoulos K, Rigopoulos D. Ustekinumab in severe complicated erythrodermic psoriasis: rapid clearing, safety, and sustained remission. Dermatol Ther. 2014 SepOct;27(5):257-9.
37. Castiñeiras I, Fernández-Diaz L, Juárez Y, Lueiro M. Sustained efficacy of ustekinumab in refractory erythrodermic psoriasis after failure of antitumor necrosis factor therapies. J Dermatol. 2012 Aug;39(8):730-1.
38.Santos-Juanes J, Coto-Segura P, Mas-Vidal A, Galache Osuna C. Ustekinumab induces rapid clearing of erythrodermic psoriasis after failure of antitumour necrosis factor therapies. Br J Dermatol. 2010 May;162(5):1144-6.
39.Wang TS, Tsai TF. Clinical experience of ustekinumab in the treatment of erythrodermic psoriasis: a case series. J Dermatol. 2011 Nov;38(11):1096-9.
40.Errichetti E, Piccirillo A: Latent tuberculosis reactivation in a patient with erythrodermic psoriasis under treatment with ustekinumab and a low dose steroid, Despite isoniazid chemoprophylaxis. Eur J Dermatol. 2014, 24(4):508-509.
41.Concha-Garzón MJ, Godoy-Trapero A, Daudén E, et al. Short- and long-term treatment of erythrodermic psoriasis with ustekinumab: A national and multicenter case series. J Am Acad Dermatol. 2014, 70(5):AB189.
42.Talat H, Wahid Z, Feroz F, Sajid M. Erythrodermic Psoriasis and Hepatitis C Infection Treated with Pegylated Interferon and Anti-TNF (Etanercept) Therapy. J Coll Physicians Surg Pak. 2017 Sep;27(9):S77-S79.
43.Romero-Maté A, García-Donoso C, Martinez-Morán C, Hernández-Núñez A, Borbujo J.Long-term management of erythrodermic psoriasis with anti-TNF agents. Dermatol Online J. 2010 Jun 15;16(6):15.
44. Esposito M, Mazzotta A, de Felice C, Papoutsaki M, Chimenti S. Treatment of erythrodermic psoriasis with etanercept. Br J Dermatol. 2006 Jul;155(1):156-9.
45. Piqué-Duran E, Pérez-Cejudo JA. [Psoriatic erythroderma treated with etanercept]. Actas Dermosifiliogr. 2007 Sep;98(7):508-10.
46.Weng HJ, Wang TS, Tsai TF. Clinical experience of secukinumab in the treatment of erythrodermic psoriasis: a case series. Br J Dermatol. 2018 Jun;178(6):1439-1440.
47.Mugheddu C, Atzori L, Lappi A, Pau M, Murgia S, Rongioletti F. Successful Secukinumab treatment of generalized pustular psoriasis and erythrodermic psoriasis. J Eur Acad Dermatol Venereol. 2017 Sep;31(9):e420-e421.
48.Mateu-Puchades A, Santos-Alarcón S, Martorell-Calatayud A, Pujol-Marco C, Sánchez-Carazo JL. Erythrodermic psoriasis and secukinumab: Our clinical experience. Dermatol Ther. 2018 Jul;31(4):e12607.
49.Galluzzo M, D’Adamio S, Campione E, Mazzilli S, Bianchi L, Talamonti M. A clinical case of severe disease burden: an erythrodermic psoriatic patient treated with secukinumab. J Dermatolog Treat. 2018 Sep 26:1-11.
50.Rongioletti F, Mugheddu C, Murgia S: Repigmentation and new growth of hairs after anti–interleukin-17 therapy with secukinumab for psoriasis. JAAD Case Rep. 2018 Jun; 4(5): 486–488.
51.Mumoli N, Vitale J, Gambaccini L, Sabatini S, Brondi B, Cei M. Erythrodermic psoriasis. QJM. 2014 Apr;107(4):315.
52.Richetta AG, Maiani E, Carlomagno V et al. Treatment of erythrodermic psoriasis in HCV+ patient with adalimumab. Dermatol Ther. 2009 Nov;22 Suppl 1:S16-8.
53.Vidal D, Peramiquel L, Olivella R, Villar M, Petiti G, González A: Erythrodermic psoriasis successfully treated with adalimumab: A case study. J Eur Acad Dermatol Venereol. 2013, 27(S4):68.
54.Saeki H, Nakagawa H, Nakajo K et al. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: Results from a 52-week, open-label, phase 3 study (UNCOVER-J). J Dermatol. 2017 Apr;44(4):355-362.
55.Lee WK, Kim GW, Hyun-Ho Cho et al. Erythrodermic Psoriasis Treated with Golimumab: A Case Report. Ann Dermatol 2015;27(4): 446-449.
56.Sano S, Kubo H, Morishima H, Goto R, Zheng R, Nakagawa H. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: Efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018 May;45(5):529-539.
57.Viguier M, Pagès C, Aubin F et al. Efficacy and safety of biologics in erythrodermic psoriasis: a multicentre, retrospective study. Br J Dermatol. 2012 Aug;167(2):417- 23.
58.Sahel H, Otsmane F, Bouadjar B: Treatment of erythrodermic psoriasis with biological therapies in two cases. J Eur Acad Dermatol Venereol. 2016, 30(S6):102.