Potential Implications for Future Therapies
Abstract
Despite recent advances in the diagnosis and treatment of multiple sclerosis, we still lack a consensus regarding the causes, pathogenesis, and mechanisms of disease progression. Current evidence indicates that multiple sclerosis is an inflammatory neurodegenerative disorder in which both adaptive and innate immunity play important roles in initiation and maintenance of the disease. Recent evidence supports the notion of molecular pathologic abnormalities beyond the plaques and dysfunction of neurons in normal appearing areas, in addition to the multifocal demyelination and axonal loss, as important features that may underlie early reversible changes in the disease. Chronic failure of remyelination, axonal regeneration, and neuronal dysfunction may contribute to disease progression. This article discusses the emerging molecular evidence for the progression of multiple sclerosis with particular focus on alterations in the local central nervous system microenvironment of neural and glial cells. The molecular pathways leading to structural and functional neurodegeneration and those that prevent regeneration need to be identified in order to design new therapeutic strategies that can halt or even reverse disease progression.
Multiple sclerosis (MS) affects 1 million people worldwide and is the leading cause of neurological disability in young adults.1 The disease has substantial personal and economic costs. It is an immune-mediated demyelinating and neurodegenerative disease of the central nervous system (CNS). Initially, more than 80% of patients experience a relapsing-remitting form of disease, characterized by exacerbations of neurologic deficits with periods of symptom remission. After several years, a high proportion of patients enter a secondary progressive phase characterized by irreversible deficits and neurodegeneration. About 10% of patients exhibit a primary progressive form of the disease from onset.1
There is substantial evidence that immune dysregulation plays an important role in the disease process in MS.2 Thus, current therapies for MS are immunomodulatory and have been effective in decreasing relapse rates but seemingly far less effective in preventing disease progression, defined as an accumulation of neurologic disability. Pathologically, neuronal and axonal loss as well as demyelination are observed in MS lesions, and they likely contribute to disease progression. In addition, evidence of remyelination can be seen in “shadow plaques”; however, a pronounced failure of remyelination occurs as the disease progresses.3-5
Although MS treatment has advanced significantly in the past 10 years, several pressing questions remain unanswered. First, there is a weak correlation between standard magnetic resonance imaging findings and clinical symptoms. In particular, there is a discrepancy between T2 lesion load and clinical disability. Some of the discrepancy has been resolved by the use of specialized magnetic resonance imaging techniques such as diffusion tensor imaging that reveal pathological changes in normal appearing white matter.6,7 In addition, differences in radiological patterns between MS subtypes may also contribute to this dissociation.7,8 To more definitively understand the evolution of MS pathologic abnormalities during the disease course, more sensitive imaging techniques and validation of these techniques by correlation of radiological with pathological findings are required.
Second, what determines the clinical outcome of benign vs malignant disease? This question requires investigation of the genetic modifiers that control immune response on the one hand and capacity for repair on the other and is currently being addressed through large-scale genetic studies. Of direct clinical relevance is the third question, that of responders vs nonresponders to therapy. This question is being addressed by several investigators through gene expression profiling of peripheral blood mononuclear cells from patients before and after start of therapy to determine the mechanisms of therapy and gene profiles that may have predictive value for response to therapy.9 Fourth, the major challenge facing clinicians today is to determine the mechanisms of disease progression and how to prevent it. Here, we focus on the emerging evidence of the contributions of resident neural cells to disease progression in MS with attention to neuroglial interactions. For reviews of the immunological aspects associated with disease progression, consult previous articles in the ARCHIVES.10,11
The current model of MS pathogenesis suggests that autoreactive T cells, B cells, myelin-specific autoantibodies, and macrophages enter the CNS and initiate demyelination and irreversible axonal loss that accumulate in chronic lesions by direct damage and in normal appearing white matter as a result of wallerian degeneration.12 However, this model alone cannot explain disease progression, given the complexities of immune-neural interaction in the CNS and the heterogeneity of pathologic abnormalities in patients with MS.13 It is likely that immune and neural dysregulation within the CNS is as critical in MS pathogenesis as the peripheral immune response and might also influence disease outcome and progression.
Evidence of Molecular Activation of Astrocytes and Microglia as “Effector” Cells in Chronic Pathogenesis in MS
Astrocytes and microglia respond to environmental cues from neural cells and immune cells, establishing a rich network of connections that can regulate the local cytokine environment during normal homeostasis and in disease. It was initially suggested that astrocytes might act as antigen presenting cells in the CNS, but in humans this role is still unclear. However, alterations in the regulation of glutamate by astrocytes are observed in MS lesions. Degradation of glutamate is mediated by glutamine synthetase and glutamine dehydrogenase, and these have been found to be reduced in MS lesions.14 Similarly, glial transporters responsible for uptake of glutamate by astrocytes are also decreased in MS lesions,14 suggesting an increase in extracellular concentration of glutamate that may be toxic to oligodendrocytes and neurons. However, the timing and the relative contribution of excitotoxicity to the overall pathological picture is not known.14,15 In addition, factors produced by astrocytes may inhibit remyelination. An example of this is Jagged, a Notch ligand, which is up-regulated after exposure to transforming growth factor ß and has been shown to reduce oligodendrocyte progenitor cell (OPC) maturation.16 However, in the model of cuprizone-mediated demyelination, Stidworthy et al17 found that the lesions remyelinate completely despite abundant expression of Jagged in glial cells and Notch in OPCs. Furthermore, ablation of Notch in OPCs did not change the rate of remyelination in this model.17 This discrepancy may relate to differences in the species studied, but the role of Jagged-Notch in inhibiting remyelination needs further study. Furthermore, astrocytes may produce cytotoxic compounds in MS. It was recently shown that astrocytes produce syncytin, a human endogenous retrovirus encoded glycoprotein that is toxic to oligodendrocytes and produces neuroinflammation.18 In addition, based on the accumulated evidence from pathological studies in humans and animal models, chronically activated parenchymal and perivascular microglia appear to be important in the disease process because complete eradication of microglia decreases substantially experimental autoimmune encephalomyelitis (EAE).19 There are numerous reports of microglia-induced neurotoxicity in vitro and evidence of microglia activation in the CNS. However, a direct role for microglia in neuronal dysfunction in vivo is less well established. Table 1 lists the molecules that have been dysregulated in microglia and astrocytes in patients with MS.
Evidence of Molecular Dysregulation of Neurons as “Afferent” Cells in Chronic Pathogenesis in MS
Neuronal cell loss and apoptosis of small numbers of neurons are present in demyelinated cortical MS lesions.20 Human T cells can induce apoptosis of human fetal neurons in vitro21; however, few T cells are seen in these areas. The degree of neuronal apoptosis observed in MS is not sufficient to explain the progression and severity of the disease, so in addition to cell death, it appears that a large number of neurons or axons in normal appearing white and gray matter in MS may also be dysfunctional. The studies supporting this notion report alteration in genes that participate in transcriptional regulation and inflammation,6,7,22-24 such as increased expression of 5-lipoxygenase and caspase 1; alterations in the distribution of sodium and calcium channels in pathological specimens,25,26 such as the N-type Ca2+ channel; and decreased expression of metabolism-related genes, such as cAMP (cyclic adenosine monophosphate) response element binding protein 1, sterol delta-7 reductase, aspartoacylase, and epsin-2.23 Thus, neurons and oligodendrocytes outside the lesions may become chronically dysfunctional and, in the presence of subtle but persistent chronic inflammation from activated glial cells and the failure of protective mechanisms,27-29 result in progressive impairment and susceptibility to structural loss of axons and cell death (Table 1). Table 2 summarizes the molecular pathways in neurons, oligodendrocytes, and progenitors that were found to be dysregulated in patients with MS.
What Are the Mechanisms That May Contribute to Neuronal and Axonal Dysfunction?
Chronic Demyelination Itself. Substantial axonal loss has been reported in the spinal cord30-33 as well as in the corpus callosum and optic nerve of patients with MS,34 and factors such as demyelination itself have been implicated. The integrity of axons is dependent in part on the integrity of the myelin sheath,35 and an intact myelin sheath protects the axon from immune-mediated damage.36 Mice lacking proteolipid protein and humans with mutation in the proteolipid protein gene develop axonal swellings and degeneration.37 Both proteolipid protein and myelin-associated glycoprotein38 are believed to be essential for delivering myelin-derived trophic signals to axons.38
Direct Damage by Inflammatory Cells. In addition to demyelination, there is strong evidence that direct interaction with inflammatory cells plays a critical role in the induction of axonal damage. The presence of macrophages/microglia as well as CD8+ T cells in MS lesions has been correlated with axonal injury.32 Soluble mediators such as complement,39 antibodies,40 and various cytokines41,42 are critical components of the immune inflammatory process and either individually or in concert have been implicated in axonal degeneration, neuronal dysfunction,43 and oligodendrocyte cell death, but some cytokines may have neuroprotective effects44 that may be exploited therapeutically. Inflammatory mediators can indirectly promote degeneration by up-regulating excitotoxic receptors on oligodendrocytes and neurons.15 Astrocyte-derived tumor necrosis factor a regulates the strength of synaptic transmission by modulating the expression of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors45 that are involved in excitotoxicity. These molecular changes are present in normal appearing white matter23,24,46 and can also be seen in normal aging brain47 and may be sustained in the absence of obvious inflammation.23 The exact role of tumor necrosis factor a is very complex; although known to be neurotoxic for neurons, oligodendrocytes, and their progenitors in vitro,48 in vivo blockade of tumor necrosis factor a resulted in worsening of MS, suggesting a yet-to-be-defined protective role of tumor necrosis factor a49 or a role in remyelination in vivo.50
Oxidative Stress and Neuronal Gene Expression. Oxidative stress may also contribute to neuronal dysfunction and axonal loss.51 Activated macrophages and microglia51 and astrocytes52 produce inducible nitric oxide synthase and nitric oxide, which are associated with oxidative damage to mitochondrial DNA in chronic active MS plaques, ultimately leading to cell dysfunction and death.53 Furthermore, experimental evidence shows that electrically active axons exposed to high concentrations of nitric oxide have enhanced susceptibility to persistent conduction block and axonal degeneration.36,54 In addition, a decrease in expression of several neuronal genes, such as synaptosomal-associated protein 25, glycine receptor, lissencephaly-related protein, ?-aminobutyric acid receptor, and Neuro D, has been reported by 2 independent groups,46,55 possibly mediated by chronic oxidative damage in susceptible genes47,53 (Table 2). Thus, reduction of oxidative stress early in the disease course may ultimately prevent chronic damage.56
Impact of Simultaneous Pathologic Abnormalities in the Progression of Disease. Our current perception that MS is a disease with 2 different temporally distinct phases, an inflammatory and a neurodegenerative phase,57 needs to be reevaluated because recent observations reveal that both phases can occur simultaneously.22,28-30N-acetyl aspartate (NAA) is a metabolite localized almost exclusively in neurons and neuronal processes in the mature brain.58 The resonance intensity of NAA therefore provides an index of neuronal integrity and can be measured by spectroscopy; a decrease in the NAA peak correlates with axonal and neuronal damage in MS and stroke.28,29 In MS, a decrease in NAA can be found early in the disease and provides evidence of early neuronal and axonal damage59 but can also be seen with alterations in neuronal metabolism without structural damage as shown in other neurological disorders.60 Consequently, abnormalities of NAA can be found in regions far from the local demyelinating areas of the brain, reflecting dysfunction of axons in projection pathways61 that may be the substrate of wallerian degeneration.22,26 Central nervous system homeostasis is maintained by local oxygen supply and pH control; it is thus not surprising that inflammation may jeopardize the delicate homeostatic balance, as described by Lassmann,62 who found ischemic-like changes in a subset of MS lesions.
Unlike stroke or other neurodegenerative diseases that have a regional preponderance, MS is characterized by multiple “hits” involving various locations within the CNS at different times. Each subsequent hit initiates focal areas of damage and more widespread areas of oxidative stress dysfunction, leading to the initiation of progression and neurodegeneration.
We suggest that the substrate for chronic neurodegeneration in MS is initiated long before any widespread structural damage. Neuronal dysfunction in normal areas characterized by metabolic and molecular changes may occur during the initial attacks.23,24,46 Because this process may be preventable, we need to learn more about this initial stage and develop therapeutic strategies to prevent irreversible damage as soon as the diagnosis of MS is made.
Impact of Aging in the Progression of Disease. An important consideration when thinking about disease progression in MS is the possibility that normal brain aging may contribute or even precipitate the onset of progression. During “normal aging,” a set of genes with central roles in synaptic plasticity, vesicular transport, and mitochondrial function have reduced expression after age 40 years.47 This is attributed to DNA damage by oxidative stress in the promoter regions, resulting in reduced expression of selectively vulnerable genes involved in learning, memory, and neuronal survival.47 Aging also has an adverse effect on remyelination, affecting recruitment and differentiation of OPCs in a model of demyelination.63 Brain aging is a risk factor for other neurodegenerative diseases, including stroke and Alzheimer disease. Thus, it seems important to study the impact of aging on neuronal function in MS. An aging effect on progression of disease may contribute to the data of Confavreux et al64 that showed variation in the time to reach an Expanded Disability Status Scale score of 4.0 among patients but consistency in the time to progress from the Expanded Disability Status Scale score 4.0 to 6.0. Data from an Italian cohort also suggests age-related onset of progression65 in that clinical disability was influenced by the patient's age. Other genes associated with inflammation and aging such as apolipoprotein E e4 have an impact on disease severity in MS.66 Thus, it is possible that normal aging and other modifier genes could have a previously unforeseen role as adjuvants of neurodegenerative changes in MS.
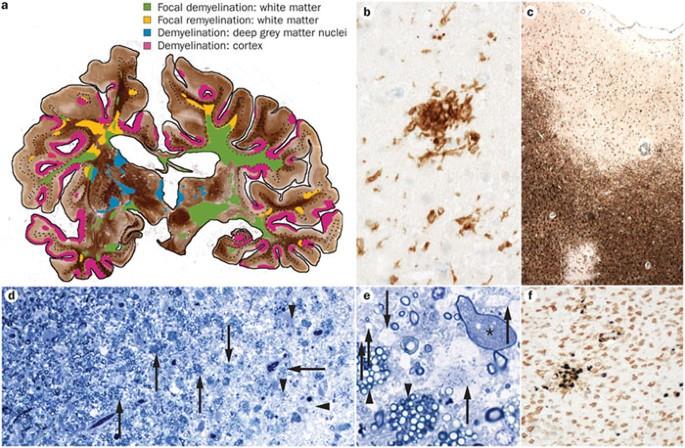
A Model for Progression. A model of the steps leading to disease progression is presented in the Figure. In this model, the initial hit mediated by immune cells and repeated bouts of inflammation results in a chronic abnormal microenvironment, leading to eventual regional compromise and brain dysfunction, which may contribute to disease irreversibility. In areas of plaques, inflammation mediated by adaptive and innate immunity initiates destruction of myelin and axons while outside the plaques, subacute and sustained inflammation is mediated by activated glia67 that establish an abnormal microenvironment. Neuronal dysfunction occurs early and a new adaptive abnormal steady state in the neuron is established, represented by alteration of gene expression and function that may be worsened by aging. A new event near the original site of pathologic abnormality (a new lesion, infection, trauma) worsens inflammation and surpasses the adaptive capacity of neurons,68 resulting in more neuronal dysfunction or cell autonomous axonal loss. New lesions in areas not previously affected multiply the regions with an abnormal microenvironment. Cell death in a dysregulated environment may further sustain inflammation by activated glia cells, resulting in a vicious cycle. At the same time, axonal loss and wallerian degeneration may contribute to distant areas of pathologic abnormality.22 Because there is heterogeneity in MS lesions, different combinations of these events may contribute to progression in MS (Figure).
Relevance to the study of neurosciencePotential Targets for Halting Progression and Increasing Regeneration
Restoring Neuronal Dysfunction. Current evidence suggests that the neurodegenerative component in MS is critical for the progression of disability.31 Thus, novel therapies for MS should target not only the peripheral immune response but also the underlying mechanisms of dysregulated CNS inflammation and neurodegeneration and should promote repair and regeneration in the CNS. There are now therapies that can prevent exacerbations, but none forestall the progression of neurodegeneration, in part because of a lack of identification of the molecular pathways that mediate the chronic alteration of neurons and surviving oligodendrocytes. Neuronal dysfunction has been described in EAE. D’Intino et al69 demonstrated a deficit in learning and memory performance in rats with EAE that correlated with a decline in choline acetyltransferase activity and nerve growth factor messenger RNA levels in the cortex, hippocampus, and basal forebrain neurons, without apparent cell loss. Furthermore, selective acetylcholinesterase inhibitors restored cognitive performance, choline acetyltransferase activity, and nerve growth factor messenger RNA expression in the rats with EAE.69 Other potential targets include neuroprotective cytokines, such as leukemia inhibitor factor and ciliary neurotrophic factor, a cytokine that promotes neuronal survival and maturation of oligodendrocytes. Ciliary neurotrophic factor–deficient mice have more severe EAE with increased oligodendrocyte apoptosis and severe vacuolar dystrophy of myelin and axonal damage.70 In addition, ciliary neurotrophic factor was reported to be neuroprotective in a model of optic neuritis,71 and leukemia inhibitor factor promoted the survival of oligodendrocytes in EAE.72
Promotion of Remyelination. We also need therapies that promote effective remyelination. Adult brain contains neural stem cells and progenitors scattered throughout the CNS in regional pools, and these cells have the capability to engage in endogenous repair. The lack of effective repair in MS suggests that either the size or number of lesions overcome the capacity of endogenous precursor cells to repair the damage or that precursor cells could themselves be targets of the inflammatory pathologic process.73
How do we boost the repair potential of neural stem cells? One approach is to manipulate the molecular signals that control the differentiation of endogenous neural stem cells and OPCs while avoiding unwanted proliferation and tumorigenesis. Some of the molecular signal candidates may include the neuroregulin glial growth factor 2 and thyroid hormone that promote oligodendrocyte progenitor maturation and remyelination in a model of MS.74,75
Another approach is through transplantation of exogenous neural stem cells, an approach that eliminates the potential problem of the endogenous cells having a genetic predisposition to malfunction. The transplantation approach offers the added options of genetically modifying the cells ex vivo prior to transplantation to optimize remyelination and support axonal repair. Regardless of the strategy, more research is needed to understand the contributions of key molecules in differentiation and survival of progenitors during MS. For instance, the transcription factors Sox-10 (SRY box family member 10), Olig-1 and Olig-2 (oligodendrocyte transcription factor 1 or 2), and Nkx2.2 (NK2 transcription factor related, locus 2) participate in the response of progenitors to demyelination. Sox-10 is required for oligodendrogenesis,76 and Nkx2.2 and Olig-2 positive cells proliferate and differentiate in response to a demyelinating insult.77 Furthermore, Olig-1 is present in MS lesions and appears to have a critical role for effective remyelination in a model of MS.78 Future work in the regulation of these genes during MS would offer ways to manipulate relevant molecular targets without inducing aberrant neurogenesis, gliogenesis, or tumorigenic proliferation.
Promotion of Axonal Regeneration. Axonal regeneration is another potential target for intervention. Injured oligodendrocytes and myelin exert negative signals for axonal regeneration; calpains (calcium-activated neutral proteinases) can degrade myelin proteins at physiological pH and are found in glia and inflammatory cells. Thus, neuronal self-repair and axonal regeneration may be impaired by negative signals released during myelin destruction. Among the products of myelinolysis, myelin-associated glycoprotein, myelin oligodendocyte glycoprotein, and Nogo inhibit axonal regeneration and are collectively called myelin-associated inhibitory factors.
Nogo is a member of the reticulon family, expressed by oligodendrocytes but not by Schwann cells, and inhibits axonal extension.79 The Nogo receptor complex, composed of the Nogo-66 receptor 1, neurotrophin p75 receptor, and LINGO-1, represses axon regeneration upon binding to myelin-associated inhibitory factors. The binding of neurotrophin to its receptor, p75 neurotrophic tyrosinekinase receptor, abolishes activation of protein kinase C and the GTPase ras homolog gene family member A and decreases neurite outgrowth.80 Nogo-66 is immunogenic and may play a role in EAE: antibodies to Nogo-66 protect from EAE,81 and Nogo-66–derived peptides are encephalitogenic while other Nogo-66 epitopes induce protective Th2 cell lines.82 Therapeutic targets to stimulate axonal regeneration include inhibitors of Nogo signaling and protein kinase C inhibitors.83
Current therapies address the inflammatory and immunological components of MS pathologic abnormalities, a strategy that is necessary to modulate the “nonpermissive environment” before contemplating neuroprotective and repair therapies. Interactions between the immune system and the CNS may lead to neurologic dysfunction but may also be necessary to initiate repair. One of the challenges in the next phase of therapeutic investigations is to dissect the positive vs negative neural-immune interactions and design selective therapies. Another challenge is how to measure the effects of neuroprotective therapies. Intrinsic challenges in MS include the relative unavailability of obtaining serial pathologic material, making it necessary to depend on novel imaging and modeling techniques.
Relevance to the practice of neurology
There is evidence that the mechanism of progression in MS is related to chronic neuronal dysfunction within the lesions and in normal appearing areas outside the lesions in both the white matter and cortex as a result of chronic inflammation, oxidative stress, and microglia activation. While this article was under review, Kutzelnigg et al84 reported a detailed study on the neuropathologic features of 52 MS cases, and their conclusions give additional support to our proposed model of progression. Therefore, the goals for MS therapy should include the following: the induction and maintenance of immunological tolerance toward self-antigens in the susceptible population, the promotion of remyelination, and the promotion of axonal regeneration but, more importantly, the prevention of axonal degeneration and neuronal dysfunction as soon as the diagnosis is made.
The current state of MS therapy has advanced significantly in the control of the inflammatory and immunologic aspects of MS that decrease relapses but are less effective in stopping progression. Therefore, we need now a concerted effort and interdisciplinary approach to study the molecular pathologic abnormalities of neural degeneration in MS to identify novel therapeutical targets.
We conclude with a quote from J. M. Charcot: “Disease is very old and nothing about it changes. It is we who change as we learn to recognize what was formerly imperceptible.”
Correspondence: Samia J. Khoury, MD, 77 Avenue Louis Pasteur, HIM R710, Boston, MA 02155 (skhoury@rics.bwh.harvard.edu).
Accepted for Publication: June 17, 2005.
Author Contributions:Study concept and design: Imitola, Chitnis, and Khoury. Acquisition of data: Imitola and Chitnis. Analysis and interpretation of data: Imitola. Drafting of the manuscript: Imitola, Chitnis, and Khoury. Critical revision of the manuscript for important intellectual content: Imitola, Chitnis, and Khoury. Statistical analysis: Imitola. Obtained funding: Khoury. Administrative, technical, and material support: Khoury. Study supervision: Imitola, Chitnis, and Khoury.
Funding/Support: This work was supported by grants from the National Institutes of Health, Bethesda, Md; the National Multiple Sclerosis Society, New York, NY; and the Nancy Davis Center Without Walls, Los Angeles, Calif.
Acknowledgment: We thank Drs Vissia Viglietta and Timothy K. Vartanian for critical review of the manuscript.
1.Noseworthy JHLucchinetti CRodriguez MWeinshenker BG Multiple sclerosis. N Engl J Med 2000;343938- 952PubMedGoogle ScholarCrossref
2.Weiner HL Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol 2004;611613- 1615
ArticlePubMedGoogle ScholarCrossref
3.Wolswijk G Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 2002;125338- 349PubMedGoogle ScholarCrossref
4.Prineas JWBarnard ROKwon EESharer LRCho ES Multiple sclerosis: remyelination of nascent lesions. Ann Neurol 1993;33137- 151PubMedGoogle ScholarCrossref
5.Chang ANishiyama APeterson JPrineas JTrapp BD NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 2000;206404- 6412PubMedGoogle Scholar
6.Traboulsee ADehmeshki JPeters KR Disability in multiple sclerosis is related to normal appearing brain tissue MTR histogram abnormalities. Mult Scler 2003;9566- 573PubMedGoogle ScholarCrossref
7.Oh JHenry RGGenain CNelson SJPelletier D Mechanisms of normal appearing corpus callosum injury related to pericallosal T1 lesions in multiple sclerosis using directional diffusion tensor and 1H MRS imaging. J Neurol Neurosurg Psychiatry 2004;751281- 1286PubMedGoogle ScholarCrossref
8.Lucchinetti CBruck WParisi JScheithauer BRodriguez MLassmann H Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000;47707- 717PubMedGoogle ScholarCrossref
9.Baranzini SEMousavi PRio J Transcription-based prediction of response to IFNbeta using supervised computational methods. PLoS Biol 2005;3e2PubMedGoogle ScholarCrossref10.Frohman EMFilippi MStuve O Characterizing the mechanisms of progression in multiple sclerosis: evidence and new hypotheses for future directions. Arch Neurol 2005;621345- 1356
ArticlePubMedGoogle ScholarCrossref
11.Frohman EMStuve OHavrdova E Therapeutic considerations for disease progression in multiple sclerosis: evidence, experience, and future expectations. Arch Neurol 2005;621519- 1530PubMedGoogle Scholar
12.Zamvil SSSteinman L Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron 2003;38685- 688PubMedGoogle ScholarCrossref
13.Wujek JRBjartmar CRicher E Axon loss in the spinal cord determines permanent neurological disability in an animal model of multiple sclerosis. J Neuropathol Exp Neurol 2002;6123- 32PubMedGoogle Scholar
14.Werner PPitt DRaine CS Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol 2001;50169- 180PubMedGoogle ScholarCrossref
15.Pitt DWerner PRaine CS Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med 2000;667- 70PubMedGoogle ScholarCrossref
16.John GRShankar SLShafit-Zagardo B Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med 2002;81115- 1121PubMedGoogle ScholarCrossref
17.Stidworthy MFGenoud SLi WW Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain 2004;1271928- 1941PubMedGoogle ScholarCrossref
18.Antony JMvan Marle GOpii W Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci 2004;71088- 1095PubMedGoogle ScholarCrossref
19.Heppner FLGreter MMarino D Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 2005;11146- 152PubMedGoogle ScholarCrossref
20.Peterson JWBo LMork SChang ATrapp BD Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 2001;50389- 400PubMedGoogle ScholarCrossref
21.Giuliani FGoodyer CGAntel JPYong VW Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol 2003;171368- 379PubMedGoogle ScholarCrossref
22.Ge YLaw MJohnson G Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 2004;201- 7PubMedGoogle ScholarCrossref
23.Lindberg RLDe Groot CJCerta U Multiple sclerosis as a generalized CNS disease: comparative microarray analysis of normal appearing white matter and lesions in secondary progressive MS. J Neuroimmunol 2004;152154- 167PubMedGoogle ScholarCrossref
24.Whitney LWBecker KGTresser NJ Analysis of gene expression in multiple sclerosis lesions using cDNA microarrays. Ann Neurol 1999;46425- 428PubMedGoogle ScholarCrossref
25.Black JADib-Hajj SBaker DNewcombe JCuzner MLWaxman SG Sensory neuron-specific sodium channel SNS is abnormally expressed in the brains of mice with experimental allergic encephalomyelitis and humans with multiple sclerosis. Proc Natl Acad Sci U S A 2000;9711598- 11602PubMedGoogle ScholarCrossref
26.Kornek BStorch MKWeissert R Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol 2000;157267- 276PubMedGoogle ScholarCrossref
27.Graumann UReynolds RSteck AJSchaeren-Wiemers N Molecular changes in normal appearing white matter in multiple sclerosis are characteristic of neuroprotective mechanisms against hypoxic insult. Brain Pathol 2003;13554- 573PubMedGoogle ScholarCrossref
28.Filippi MBozzali MRovaris M Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain 2003;126433- 437PubMedGoogle ScholarCrossref
29.Ge YGonen OInglese MBabb JSMarkowitz CEGrossman RI Neuronal cell injury precedes brain atrophy in multiple sclerosis. Neurology 2004;62624- 627PubMedGoogle ScholarCrossref
30.Ferguson BMatyszak MKEsiri MMPerry VH Axonal damage in acute multiple sclerosis lesions. Brain 1997;120393- 399PubMedGoogle ScholarCrossref
31.Trapp BDPeterson JRansohoff RMRudick RMork SBo L Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998;338278- 285PubMedGoogle ScholarCrossref
32.Bitsch ASchuchardt JBunkowski SKuhlmann TBruck W Acute axonal injury in multiple sclerosis: correlation with demyelination and inflammation. Brain 2000;1231174- 1183PubMedGoogle ScholarCrossref
33.Ganter PPrince CEsiri MM Spinal cord axonal loss in multiple sclerosis: a post-mortem study. Neuropathol Appl Neurobiol 1999;25459- 467PubMedGoogle ScholarCrossref
34.Evangelou NKonz DEsiri MMSmith SPalace JMatthews PM Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 2000;1231845- 1849PubMedGoogle ScholarCrossref
35.Brady STWitt ASKirkpatrick LL Formation of compact myelin is required for maturation of the axonal cytoskeleton. J Neurosci 1999;197278- 7288PubMedGoogle Scholar
36.Redford EJKapoor RSmith KJ Nitric oxide donors reversibly block axonal conduction: demyelinated axons are especially susceptible. Brain 1997;1202149- 2157PubMedGoogle ScholarCrossref
37.Garbern JYYool DAMoore GJ Patients lacking the major CNS myelin protein, proteolipid protein 1, develop length-dependent axonal degeneration in the absence of demyelination and inflammation. Brain 2002;125551- 561PubMedGoogle ScholarCrossref
38.Yin XCrawford TOGriffin JW Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci 1998;181953- 1962PubMedGoogle Scholar
39.Mead RJSinghrao SKNeal JWLassmann HMorgan BP The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol 2002;168458- 465PubMedGoogle ScholarCrossref
40.Linington CMorgan BPScolding NJWilkins PPiddlesden SCompston DA The role of complement in the pathogenesis of experimental allergic encephalomyelitis. Brain 1989;112895- 911PubMedGoogle ScholarCrossref
41.Chavany CVicario-Abejon CMiller GJendoubi M Transgenic mice for interleukin 3 develop motor neuron degeneration associated with autoimmune reaction against spinal cord motor neurons. Proc Natl Acad Sci U S A 1998;9511354- 11359PubMedGoogle ScholarCrossref
42.Campbell ILAbraham CRMasliah E Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A 1993;9010061- 10065PubMedGoogle ScholarCrossref
43.Alcazar ARegidor IMasjuan JSalinas MAlvarez-Cermeno JC Axonal damage induced by cerebrospinal fluid from patients with relapsing-remitting multiple sclerosis. J Neuroimmunol 2000;10458- 67PubMedGoogle ScholarCrossref
44.Brewer KLBethea JRYezierski RP Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp Neurol 1999;159484- 493PubMedGoogle ScholarCrossref
45.Beattie ECStellwagen DMorishita W Control of synaptic strength by glial TNFalpha. Science 2002;2952282- 2285PubMedGoogle ScholarCrossref
46.Ibrahim SMMix EBottcher T Gene expression profiling of the nervous system in murine experimental autoimmune encephalomyelitis. Brain 2001;1241927- 1938PubMedGoogle ScholarCrossref
47.Lu TPan YKao SY Gene regulation and DNA damage in the ageing human brain. Nature 2004;429883- 891PubMedGoogle ScholarCrossref
48.Andrews TZhang PBhat NR TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res 1998;54574- 583PubMedGoogle ScholarCrossref
49.Lenercept Multiple Sclerosis Study Group and University of British Columbia MS/MRI Analysis Group, TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 1999;53457- 465PubMedGoogle ScholarCrossref
50.Arnett HAMason JMarino MSuzuki KMatsushima GKTing JP TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 2001;41116- 1122PubMedGoogle ScholarCrossref
51.Brosnan CFBattistini LRaine CSDickson DWCasadevall ALee SC Reactive nitrogen intermediates in human neuropathology: an overview. Dev Neurosci 1994;16152- 161PubMedGoogle ScholarCrossref
52.Vladimirova OO’Connor JCahill AAlder HButunoi CKalman B Oxidative damage to DNA in plaques of MS brains. Mult Scler 1998;4413- 418PubMedGoogle ScholarCrossref
53.Lu FSelak MO’Connor J Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 2000;17795- 103PubMedGoogle ScholarCrossref
54.Smith KJKapoor RHall SMDavies M Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol 2001;49470- 476PubMedGoogle ScholarCrossref
55.Lock CHermans GPedotti R Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med 2002;8500- 508PubMedGoogle ScholarCrossref56.Kalkers NFBarkhof FBergers Evan Schijndel RPolman CH The effect of the neuroprotective agent riluzole on MRI parameters in primary progressive multiple sclerosis: a pilot study. Mult Scler 2002;8532- 533PubMedGoogle ScholarCrossref
57.Steinman L Multiple sclerosis: a two-stage disease. Nat Immunol 2001;2762- 764PubMedGoogle ScholarCrossref
58.Simmons MLFrondoza CGCoyle JT Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 1991;4537- 45PubMedGoogle ScholarCrossref
59.De Stefano NNarayanan SFrancis GS Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol 2001;5865- 70
ArticlePubMedGoogle ScholarCrossref
60.Li LMCendes FAndermann FDubeau FArnold DL Spatial extent of neuronal metabolic dysfunction measured by proton MR spectroscopic imaging in patients with localization-related epilepsy. Epilepsia 2000;41666- 674PubMedGoogle ScholarCrossref
61.De Stefano NNarayanan SMatthews PMFrancis GSAntel JPArnold DL In vivo evidence for axonal dysfunction remote from focal cerebral demyelination of the type seen in multiple sclerosis. Brain 1999;1221933- 1939PubMedGoogle ScholarCrossref
62.Lassmann H Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci 2003;206187- 191PubMedGoogle ScholarCrossref
63.Sim FJZhao CPenderis JFranklin RJ The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 2002;222451- 2459PubMedGoogle Scholar
64.Confavreux CVukusic SMoreau TAdeleine P Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000;3431430- 1438PubMedGoogle ScholarCrossref
65.Liguori MMarrosu MGPugliatti M Age at onset in multiple sclerosis. Neurol Sci 2000;21(suppl 2)S825- S829PubMedGoogle ScholarCrossref
66.Enzinger CRopele SSmith S Accelerated evolution of brain atrophy and “black holes” in MS patients with APOE-epsilon 4. Ann Neurol 2004;55563- 569PubMedGoogle ScholarCrossref
67.Nishie MMori FOgawa M Multinucleated astrocytes in old demyelinated plaques in a patient with multiple sclerosis. Neuropathology 2004;24248- 253PubMedGoogle ScholarCrossref
68.Clarke GCollins RALeavitt BR A one-hit model of cell death in inherited neuronal degenerations. Nature 2000;406195- 199PubMedGoogle ScholarCrossref
69.D'Intino GParadisi MFernandez M Cognitive deficit associated with cholinergic and nerve growth factor down-regulation in experimental allergic encephalomyelitis in rats. Proc Natl Acad Sci U S A 2005;1023070- 3075PubMedGoogle ScholarCrossref
70.Linker RAMaurer MGaupp S CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med 2002;8620- 624PubMedGoogle ScholarCrossref
71.Maier KRau CRStorch MK Ciliary neurotrophic factor protects retinal ganglion cells from secondary cell death during acute autoimmune optic neuritis in rats. Brain Pathol 2004;14378- 387PubMedGoogle ScholarCrossref
72.Butzkueven HZhang JGSoilu-Hanninen M LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med 2002;8613- 619PubMedGoogle ScholarCrossref
73.Imitola JSnyder EYKhoury SJ Genetic programs and responses of neural stem/progenitor cells during demyelination: potential insights into repair mechanisms in multiple sclerosis. Physiol Genomics 2003;14171- 197PubMedGoogle Scholar
74.Cannella BHoban CJGao YL The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Natl Acad Sci U S A 1998;9510100- 10105PubMedGoogle ScholarCrossre
f75.Calza LFernandez MGiuliani AAloe LGiardino L Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A 2002;993258- 3263PubMedGoogle ScholarCrossref
76.Stolt CCRehberg SAder M Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev 2002;16165- 170PubMedGoogle ScholarCrossref
77.Fancy SPZhao CFranklin RJ Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci 2004;27247- 254PubMedGoogle ScholarCrossref
78.Arnett HAFancy SPAlberta JA bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 2004;3062111- 2115PubMedGoogle ScholarCrossref
79.GrandPre TNakamura FVartanian TStrittmatter SM Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 2000;403439- 444PubMedGoogle ScholarCrossref
80.Yamashita TTucker KLBarde YA Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 1999;24585- 593PubMedGoogle ScholarCrossref
81.Karnezis TMandemakers WMcQualter JL The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat Neurosci 2004;7736- 744PubMedGoogle ScholarCrossref
82.Fontoura PHo PPDeVoss J Immunity to the extracellular domain of Nogo-A modulates experimental autoimmune encephalomyelitis. J Immunol 2004;1736981- 6992PubMedGoogle ScholarCrossref
83.Sivasankaran RPei JWang KC PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci 2004;7261- 268PubMedGoogle ScholarCrossref
84.Kutzelnigg ALucchinetti CFStadelmann C Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005;1282705- 2712PubMedGoogle ScholarCrossref